The FDA Made the Right Call. It Still Hurts.
I hate shots. You hate shots. But if all that separated us and the end of the pandemic was just one more dose of the COVID vaccine, we’d all be proffering up our arms to the scientists at Pfizer and Moderna, hoping to catch a single drop of the magical formula that promised to save everyone.
Regrettably, this will not come to pass. So said the FDA when, two weeks ago, they rejected emergency authorization of booster shots for anyone not over the age of 65 or at high risk of infection. That decision may come as a surprise to some, considering the ubiquity of annual flu shots and how recent trials have indicated high booster effectiveness against COVID. Even if a third prick did not provide lifetime immunity, wouldn’t it help flatten the still-positive trendlines of new cases, hospitalizations, and deaths in the United States?
Vaccine companies justify the booster shot by pointing to patients’ gradual decrease in antibodies (the body’s powerful but short-lived defense that blocks COVID-19) in the months after vaccination. As this initial protection weakens, breakthrough infections may occur. However, vaccinated individuals are left with other long-term protections that make them ten times less likely to be hospitalized than unvaccinated individuals, raising the question of whether booster shots could actually save more lives in non-compromised groups.
Regardless of the science, boosters’ viability has also emerged as a debate over who gets shots and when. Billions of people across the globe continue to lack access to the basic two-dose vaccines that are widely available in first-world nations; according to the New York Times’ World Vaccination Tracker, more than 80 countries had under 20% of their populations fully protected as of September 24.
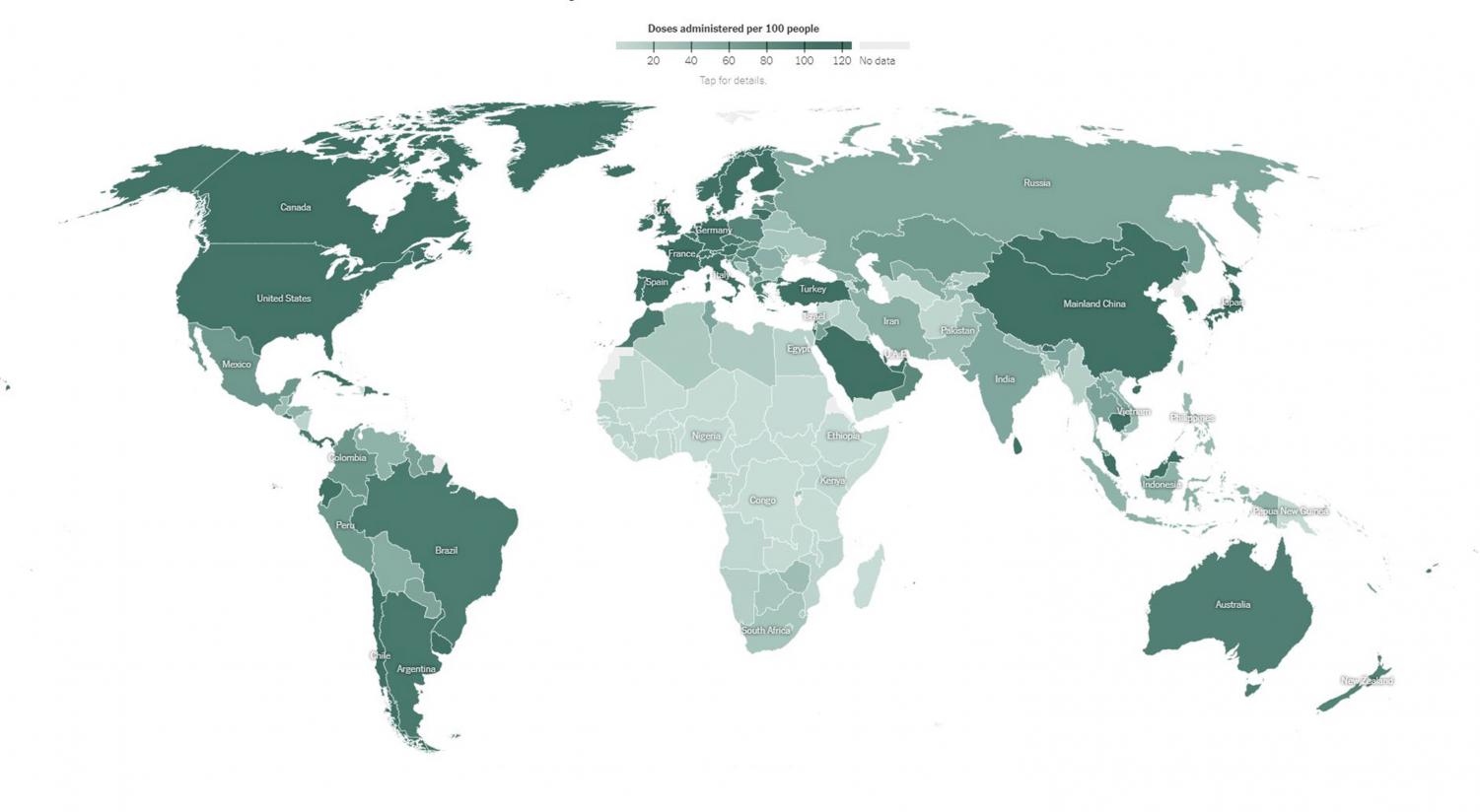
Perhaps it would be easier for us to take the booster and forget about COVID for a few glorious months of maskless school, sports, and music-making. Yet even if third doses were authorized for students, the inequalities of global vaccine distribution would remain. “There should be more attention focused on getting the two-dose vaccine to the rest of the world,” asserts Stella D. ’24. Prioritizing ourselves over those who have no immunity at all would be “selfish,” since we already have far higher protection against severe cases.
On a grander scale, Upper School Bioethics Teacher Kathryn Brooks wonders, “Do we have a duty, as a wealthy industrialized nation, to use some of our resources to help people outside our borders?… [What] if it detracts from the safety or well-being of American citizens?” In light of the FDA’s decision, vaccine supply not reserved for boosters could potentially be donated to countries in need. Among Lakesiders and top scientists and officials, there seems to be a growing consensus that such a plan would be more equitable and worthwhile than if vaccinated individuals simply received third doses.
But the FDA’s rejection of booster shots for the young and immunocompetent calls into question how the pandemic will ever end for us. Without the additional protection against breakthrough infections, cases in vaccinated populations will continue to rise even as hospitalizations flatline. At the same time, these infections will be milder than what they might have been just one or two years ago.
For its part, the Lakeside administration acknowledges that reaching zero cases is “impossible” and, as COVID-19 Health and Safety Officer Bryan Smith states, our response should focus on how to “detect a positive case and quickly address it before it has the opportunity to spread.” Still, questions remain: how long will COVID-19 present such danger as to require weekly testing and daily contact tracing? How long before the virus’ status passes from deadly global pandemic to seasonal flu? Before we stop fretting over every new infection and only need worry about the most severe cases? Only time will tell.
Hailing from Columbia, Missouri, Minoo is defined by his passion for playing the oboe and writing journalistic prose. With Hugo, his 9-year-old cockapoo,...
